Water Vs Acetone: Testing Working Fluid Options
|
|
Share this page with your friends:
|
The information presented below was collected to compare the performance of water vs. acetone as latent heat transfer fluids for solar collector heat pipe applications.
Introduction/Hypothesis:
Water is generally a cheap and accessible resource with a boiling point at atmospheric pressure that's close to but not quite within the range of average working temperatures for solar collectors, and has the potential to freeze and cause damage within the pipe; a frozen working fluid would also postpone the heat transfer cycle until it thaws first, which would have a negative effect on overall performance. Acetone is also a generally cheap and accessible resource though not near as much as water and is extremely flammable, but it has a boiling point that's close to half in comparison (approx 56°C at atmospheric pressure) which is a suitable phase transition temperature for solar collectors, and has a melting point of -90°C so it's far less likely to freeze even in the coldest climates.
If a partial vacuum can be achieved within the water heat pipe by heating it to a boil and relying on the rising vapour to expel the air (a non-condensing fluid that would interfere with performance) from the pipe prior to capping and sealing, then the boiling point should be lowered to within the working temperature range of solar collectors and be comparable to that of acetone. Acetone is miscible with water, and a mixture of both could also yield an ideal solution for solar thermal collectors that has a suitably low working temperature with freeze resistant qualities.
Water is generally a cheap and accessible resource with a boiling point at atmospheric pressure that's close to but not quite within the range of average working temperatures for solar collectors, and has the potential to freeze and cause damage within the pipe; a frozen working fluid would also postpone the heat transfer cycle until it thaws first, which would have a negative effect on overall performance. Acetone is also a generally cheap and accessible resource though not near as much as water and is extremely flammable, but it has a boiling point that's close to half in comparison (approx 56°C at atmospheric pressure) which is a suitable phase transition temperature for solar collectors, and has a melting point of -90°C so it's far less likely to freeze even in the coldest climates.
If a partial vacuum can be achieved within the water heat pipe by heating it to a boil and relying on the rising vapour to expel the air (a non-condensing fluid that would interfere with performance) from the pipe prior to capping and sealing, then the boiling point should be lowered to within the working temperature range of solar collectors and be comparable to that of acetone. Acetone is miscible with water, and a mixture of both could also yield an ideal solution for solar thermal collectors that has a suitably low working temperature with freeze resistant qualities.
|
Methods:
The pipes were constructed from 3/8" diameter copper cut to approximately 20" long each. The condensers were made using 3/8"-1/2" brass adapters with a 1.5" long copper pipe and plug soldered into one end; this allowed for easy assembly and disassembly of the pipes to make any necessary adjustments during testing and eliminated any potential risk of fire with a soldering torch being near the vapours that escape from the top of the acetone pipe as it's being sealed. The evaporator ends were simply clamped and soldered prior to the fill and final assembly. The inside of each pipe was cleaned thoroughly with isopropyl alcohol and distilled water, then 20% of their volumes filled with either water or acetone. A heat source was then applied to the evaporator sections to force the working fluids to vaporize and expel the air from the pipe. Before the heat was removed, the pipes were capped and sealed to ensure that decreasing vapour pressure wouldn't allow air back in. Prior to each test the working fluid in each pipe was replaced and the pipes were resealed. |
For performance logging, the evaporators were placed vertically in a steel pot containing 2L of water that was gradually heated with a 1.5 kW resistance heater (water temp beginning at ambient room temp and ending at ~100°C) and the pipes were positioned at an angle of approximately 80°. Temperature data was collected from each end of each pipe via four NTC sensors taped to the pipe surface, then logged and graphed for a comparison of phase transition temperatures using PC software.
|
Note: a vacuum is not needed for a heat pipe to function properly - all that is required is expelling any non-condensing gases from the pipe before sealing it and using a working fluid with a suitable phase change temperature. For this reason a partial vacuum will be created in the water pipe simply to help lower it's boiling point, but not in the acetone pipe as it already has an acceptable normal phase transition temperature which should be demonstrated by the results of this research.
Results:
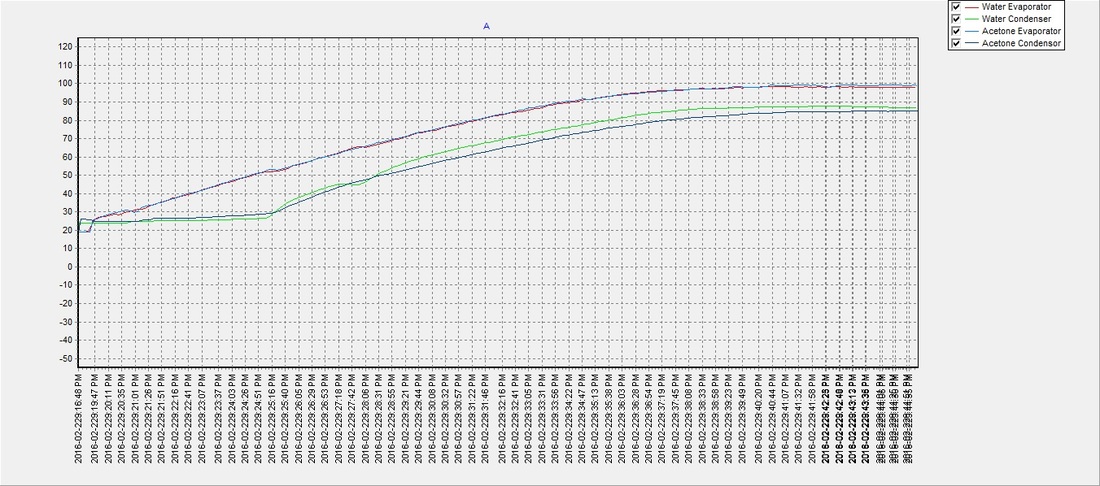
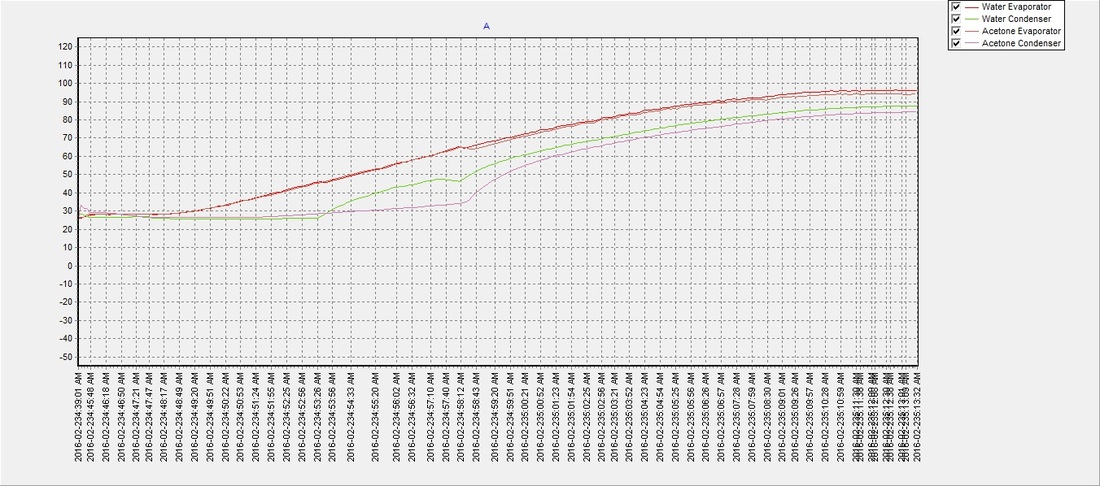
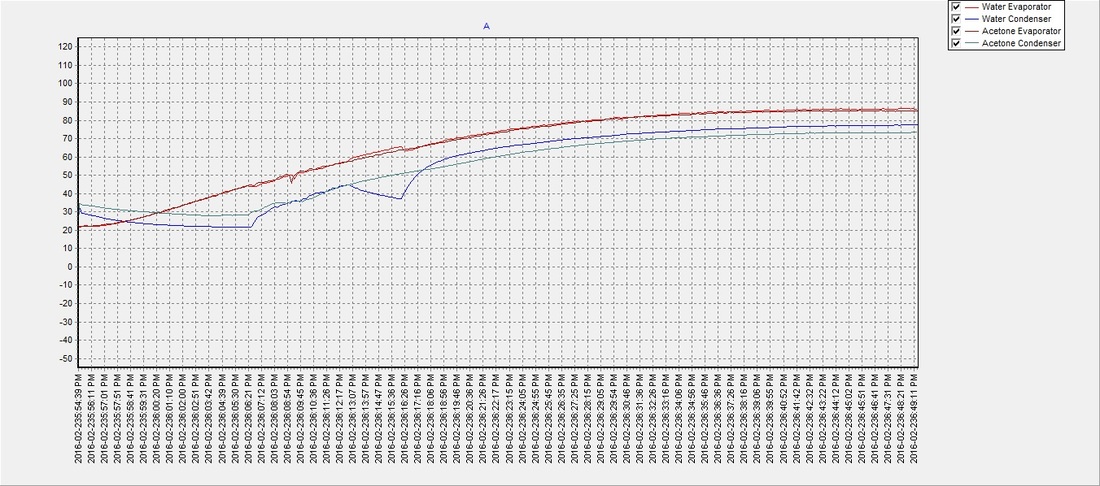
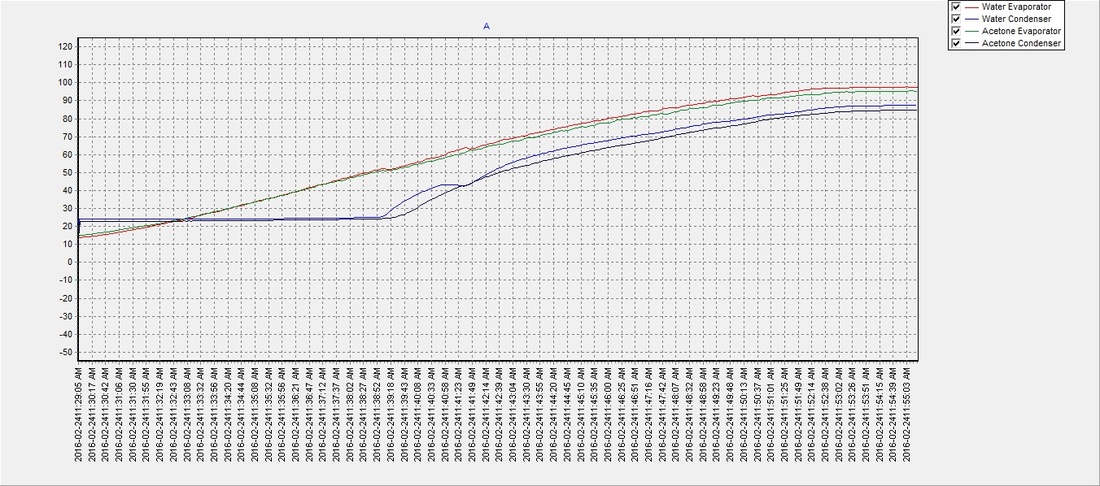
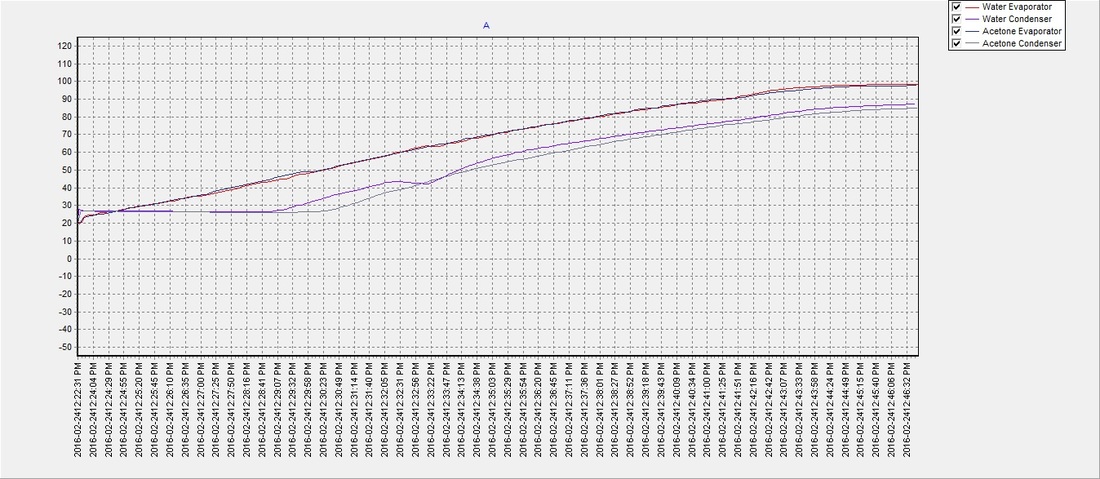
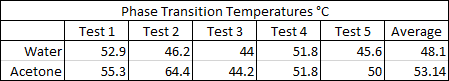
There seemed to be an issue with the water pipe when the evaporator reached approx. 60°C and the condenser temperature would drop for a few moments but then recover. It was never determined what the cause was, but despite the anomaly the water (on average) reached a phase transition temperature slightly sooner than the acetone with an average of approximately 48°C. The results suggest that a partial vacuum (~12 psi below atmospheric pressure) can be achieved using the previously described method, to lower water's boiling point and make it an efficient and safer choice over acetone as a solar thermal working fluid. The partial vacuum may also allow for expansion when the water freezes and potentially eliminate any risk of damage to the pipe, which will be determined in future tests:
Results:
There seemed to be an issue with the water pipe when the evaporator reached approx. 60°C and the condenser temperature would drop for a few moments but then recover. It was never determined what the cause was, but despite the anomaly the water (on average) reached a phase transition temperature slightly sooner than the acetone with an average of approximately 48°C. The results suggest that a partial vacuum (~12 psi below atmospheric pressure) can be achieved using the previously described method, to lower water's boiling point and make it an efficient and safer choice over acetone as a solar thermal working fluid. The partial vacuum may also allow for expansion when the water freezes and potentially eliminate any risk of damage to the pipe, which will be determined in future tests:
Water Vs Acetone Performance Data
|
Test 1
|
Test 2
|
Test 3
|
Test 4
|
Test 5
| ||||||||||
To determine a suitable antifreeze mixture for heat pipes with water as working fluids in very little to no vacuum, four 8 ml solutions were mixed in separate containers using a 7:1, 6:2, 5:3, and 4:4 water to acetone ratio, then sealed and left in a freezer with an ambient temperature of -4°F/-20°C for approximately 24 hours. When removed it was determined that as expected, the 7:1 solution was almost completely solid and the 4:4 solution was the only mixture that remained completely in liquid state. The 4:4 solution will be used in a future comparison with water as a working fluid via a series of heat transfer tests conducted identically to the previously described 'water vs acetone' tests.
|
Water/Acetone Solutions After 24hr Exposure To -20°C/-4°F Environment
|
|